Demonstrated PFS results, including long-term follow up

Demonstrated PFS results, including long-term follow up
FOTIVDA: Superior results vs sorafenib1
- 44% improvement in median PFS (5.6 months vs 3.9 months)
- 27% reduction in risk of death or progression (HR=0.73)
mPFS: 5.6 months with FOTIVDA vs 3.9 months with sorafenib (HR=0.73 [95% CI: 0.56, 0.95], P=0.02)

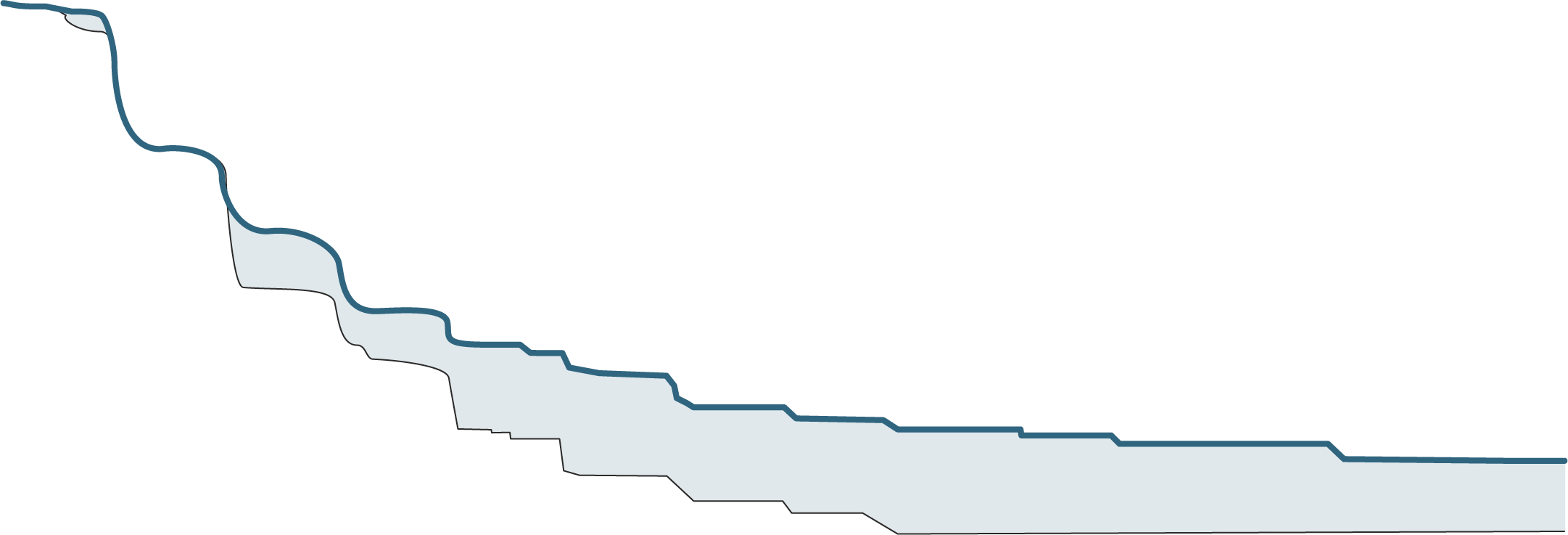


FOTIVDA: Long-term PFS from 5-year follow-up2
- Landmark data show PFS rates are consistently higher with FOTIVDA vs sorafenib, with 12% vs 2% and 8% vs 0% at three and four years, respectively
Progression Free

- LT-PFS data is based on exploratory analysis of investigator-assessed PFS, with a study start date of May 2016 and a date cutoff of May 24, 20211,2
- Investigator-assessed PFS analysis upon extended follow-up was consistent with the independent review committee PFS analysis, which was the primary endpoint for TIVO-32
- The safety profile of FOTIVDA was consistent with the full Prescribing Information
*Open-label, multicenter study in North America and the European Union. Primary endpoint: PFS assessed by blinded independent review committee. Secondary endpoint: ORR (defined as CR+PR), DOR, OS, tolerability, and safety.
Primary PFS endpoints for FOTIVDA
FOTIVDA is the first and only VEGFR TKI with long-term PFS data in 3L RCC treatment.
Primary PFS endpoints for FOTIVDA
See Dr. Pedro C Barata, Director of GU Medical Oncology Research Program, University Hospitals Seidman Cancer Institute, talk about the value of FOTIVDA’s efficacy data.
FOTIVDA is the first and only TKI with long-term PFS data in 3L RCC treatment.
Watch a discussion of the 3- and 4-year PFS data with Dr. Pedro C Barata in this short informative video.
See PFS analysis by subgroup.
Explore safety and tolerability.
Reach out to an AVEO
Oncology Account
Manager.
CI=confidence interval; CR=complete response; DOR=duration of response; HR=hazard ratio; LT=long-term; mPFS=median progression-free survival; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PR=partial response.
References: 1. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95-104. 2. Beckermann KE, Asnis-Alibozek AG, Atkins MB, et al. Long-term survival in patients with relapsed/refractory advanced renal cell carcinoma treated with tivozanib: analysis of the phase III TIVO-3 trial. Oncologist. 2024;29(3):254-262.